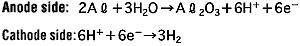2011-3-29 20:16:53
views
Electrolytic capacitance values are not as tightly-specified as with bulk dielectric capacitors. Especially with aluminum electrolytic, it is quite common to see an electrolytic capacitor specified as having a "guaranteed minimum value" and no upper bound on its value. For most purposes (such as power supply filtering and signal coupling), this type of specification is acceptable.
jb Capacitors is a professional manufacturer for aluminum electrolytic capacitors. We have around 30 years manufacturing experience of electrolytic capacitors. Generally speaking, jb Capacitors focuses on large can aluminum electrolytic capacitors and SMD aluminum electrolytic capacitors. All these types of Aluminum Electrolytic Capacitors are detailed introduced in our website: www.jbcapacitors.com.
2011-3-25 12:26:32
views
In aluminum electrolytic capacitors, the layer of insulating aluminum oxide on the surface of the aluminum plate acts as the dielectric, and it is the thinness of this layer that allows for a relatively high capacitance in a small volume. This oxide has a dielectric constant of 10, which is several times higher than most common polymer insulators. This layer can withstand an electric field strength of the order of 25 megavolts per meter which is significant fraction of that of common polymers. This combination of high capacitance and reasonably high voltage result in high energy density.
2011-3-22 17:7:22
views
Aluminum is used for the electrodes by using a thin oxidization membrane.
Large values of capacitance can be obtained in comparison with the size of the capacitor, because the dielectric used is very thin.
The most important characteristic of electrolytic capacitors is that they have polarity. They have a positive and a negative electrode.[Polarised] This means that it is very important which way round they are connected. If the capacitor is subjected to voltage exceeding its working voltage, or if it is connected with incorrect polarity, it may burst. It is extremely dangerous, because it can quite literally explode. Make absolutely no mistakes.
Generally, in the circuit diagram, the positive side is indicated by a "+" (plus) symbol.
2011-3-19 2:20:11
views
In aluminum electrolytic capacitors, the layer of insulating aluminum oxide on the surface of the aluminum plate acts as the dielectric, and it is the thinness of this layer that allows for a relatively high capacitance in a small volume. This oxide has a dielectric constant of 10, which is several times higher than most common polymer insulators. It can withstand an electric field strength of the order of 25 megavolts per meter which is an acceptable fraction of that of common polymers. This combination of high capacitance and reasonably high voltage result in high energy density.
Most electrolytic capacitors are polarized and require one of the electrodes to be positive relative to the other; they may catastrophically fail if voltage is reversed. This is because a reverse-bias voltage above 1 to 1.5 V will destroy the center layer of dielectric material via electrochemical reduction. Following the loss of the dielectric material, the capacitor will short circuit, and with sufficient short circuit current, the electrolyte will rapidly heat up and either leak or cause the capacitor to burst, often in spectacularly dramatic fashion.
2011-3-9 11:43:5
views
The following rules should be observed when handling aluminum electrolytic capacitors:
Any escaping electrolyte should not come into contact with eyes or skin.
If electrolyte does come into contact with the skin, wash the affected parts immediately with running water. If the eyes are affected, rinse them for 10 minutes with plenty of water. If symptoms persist, seek medical treatment.
...
2011-2-9 23:6:4
views
Aluminum is an abundant metallic chemical element which is widely used throughout the world for a wide range of products. Many consumers interact with some form of aluminum on a daily basis, especially if they are active in the kitchen.
The element has an atomic number of 13, and it is identified with the symbol Al on the periodic table of elements.
It is classified in the poor metals, sharing the property of extreme malleability with metals like tin and lead.
2011-1-29 21:27:56
views
Round Feed Through capacitors, in a skin tight plastic wrap with solderable ends (Style Q). The hole in the center of the capacitor is either a Teflon Tube, Paper Tube or Phenolic Tube ranging in sizes from just a wire to a large threaded stud to fit through. The majority of Feed Throughs are custom made to exact customer requirements. They are very popular in filter applications.

2011-1-21 2:4:52
views
[2]-3 Electrolyte
Aluminum electrolytic capacitors are made by layering the electrolytic paper between the anode and cathode foils, and then coiling the result. The process of preparing an electrode facing the etched anode foil surface is extremely difficult. Therefore, the opposing electrode is created by filling the structure with an electrolyte. Due to this process, the electrolyte essentially functions as the cathode. The basic functional requirements for the electrolyte are as follows:
- (1) Chemically stable when it comes in contact with materials used in the anode, cathode, and electrolytic paper.
- (2) Easily wets the surfaces of the electrode.
- (3) Electrically conductive.
- (4) Has the chemical ability to protect the anode oxide thin film and compensate for any weaknesses therein.
- (5) Low volatility even at high temperatures.
- (6) Long-term stability and characteristics that take into consideration such things as toxicity.
2011-1-20 1:52:14
views
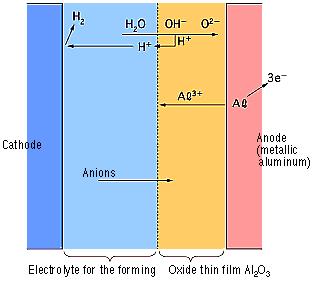
[2]-2 Forming (Anode Oxidation)
The "Forming" process is defined by creating an electrically insulating oxide (to provide the withstand voltage) on the aluminum surface by performing anode oxidation in the electrolytic solution used for the growth. The produced chemical film is used as the anode thin film.
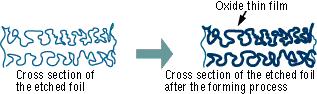
The anode oxidation, as follow shown, is produced by applying a voltage to the submerged foil found in the electrolytic solution used for growing the oxide film. Generally, the electrolytic solution is an aqueous solution such as ammonium boric acid, ammonium phosphate, or ammonium adipic for acid.
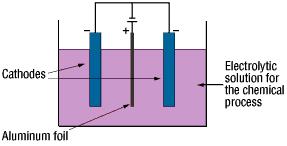
During the anode oxidation (DC electrolysis), AL2O3 is produced by a reaction between the water and the aluminum's Al3+ ions. The thickness of the grown thin film is nearly proportional to the applied voltage] with approximately 1.0 to 1.4 nm per volt. The chemical reactions on the anode side and the cathode side are as follows.
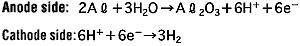
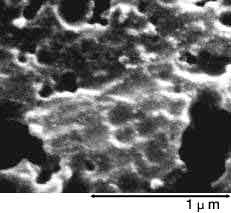
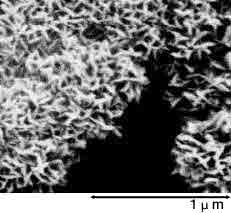
Below photographs show a magnified view of the oxide layer produced through anode oxidation.
| Low-voltage forming foil | High-voltage forming foil |
| 
| 
|
| Photograph of surface | Photograph of surface |
2011-1-19 18:58:59
views
[2]-1 Surface Roughing (Etching)
The raw foil for the anode uses a high-purity aluminum foil (a minimum purity level of 99.99%) that is normally 50 to 100 m thick. The cathode foil material uses an aluminum foil that is at least 99% pure and about 15 to 60 m thick. Because the capacitance is proportional to the surface area of the electrodes, the effective surface area is increased by roughening (etching) the surface of the aluminum foil before growing the dielectric film. Generally, this surface roughening is referred to as "etching."
There are two typical etching processes. The first option submerges the aluminum foil in hydrochloric acid (physical etching). A secondary option is electrolysis where the aluminum as the anode is placed in an aqueous hydrochloric acid solution (electrochemical etching). In electrochemical etching, the etching profile will vary depending on factors such as the waveform of the electrical current, the composition of the solution, and the temperature. The etching method can be determined by the desired capacitor performance. Generally, it is possible to achieve etching multipliers (the ratio between the surface area of the smooth foil and the effective surface area of the etched foil) approximately between 3 and 120.
The foil is then rinsed thoroughly with water. Any residual chlorine ions on the foil's surface after etching can corrode the foil and damage the capacitor. After etching, the foil's surface can be categorized broadly as shown below by the selected voltage at which the capacitor functions properly. See the magnified view of the surface in below photograph.
| | Foil Surface
(3500x Magnification) | Cross-section of Capacitor
(350x Magnification) |
Types of
Etched Foils | Low-voltage foil |  |  |
| High-voltage foil |  |  |